Mikrowelt: Bose-Einstein-Kondensat/Photonenstatistik
Rydberg Atoms ...
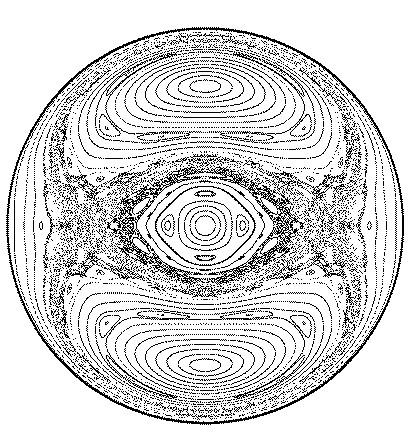
... are suited for studies into the transition between classical and quantum mechanics, since they have one or more electrons in highly excited states (main quantum number n) - according to the correspondence principle thus
closeto classical orbits.
Under the influence of external fields even the hydrogen electron may exhibit chaotic behaviour in the classical limit.
The figure shows a cross section through the classical phase space of the electron for a highly excited hydrogen atom in a magnetic field (which causes a toroidal motion). The electron posesses a mixed
phase space, with coexisting regular and chaotic motions.
[ Sitemap ]
[ info ] This website was created by the MPI for the History of Science.
 Scene
Scene


 1st Slide
1st Slide
 Branching Point
Branching Point
 Module: Mikrowelt: Bose-Einstein-Kondensat/Photonenstatistik
Module: Mikrowelt: Bose-Einstein-Kondensat/Photonenstatistik Sequence: lev0_start
Sequence: lev0_start Branching Point: Particles and Light under Control
Branching Point: Particles and Light under Control Slide: Functional Principle of the Paul Trap
Slide: Functional Principle of the Paul Trap Back
Back

