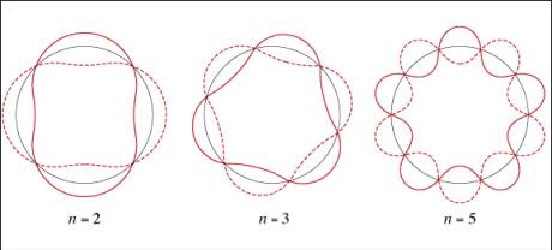
For graphical reasons the figure shows the orbits equally large, which results in decreasing wavelengths with increasing n. Oppositely, the orbits should be drawn increasingly large and the wavelength remain constant. Electronic orbits with a high quantum number are stable only under very cold conditions. Such giant atoms, called Rydberg atoms, may be generated today. A hydrogen atom with quantum number n=600 is already as large as 20 microns. Among other reasons, these exots are of interest since they make the quantum-classical boundary domain accessible to direct observation.
Unfortunately, Bohr's plausible, even if physically not completely conclusive atomic model does not properly apply to atoms with more than one electron. Whereas the radiation spectrum of hydrogen may well be explained this way, this holds no longer even for helium (two electrons).
 Scene
Scene


 1st Slide
1st Slide
 Branching Point
Branching Point
 Module: Mikrowelt: Bose-Einstein-Kondensat/Photonenstatistik
Module: Mikrowelt: Bose-Einstein-Kondensat/Photonenstatistik Sequence: lev0_start
Sequence: lev0_start Branching Point: Particles and Light under Control
Branching Point: Particles and Light under Control Slide: Functional Principle of the Paul Trap
Slide: Functional Principle of the Paul Trap Back
Back

